


|
Taking it further: |
|
1. Tell the students to try some other liquids or solutions like soda or baking powder in water or sugar in water and try to compare them. 2. Try to use different kinds of charcoal. 3. Ask the student on how they can increase the voltage of the improvised battery. |
|
1. Mix salt and water in a drinking cup to make a saltwater solution. Make sure that it is saturated that you can see salt settling in the bottom of the cup after mixing it. |
|
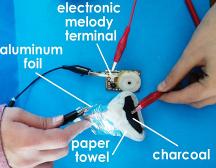
2. Soak the paper towel into the saltwater solution. Use the soaked paper towel to cover the charcoal. Then, cover it with an aluminum foil as shown in the picture. This is ready to use as a battery. |
|
3. To check if the battery works, get a pair of connecting wire with alligator clip and connect it to the terminals of the electronic melody. Then touch one end of the alligator clip to the charcoal and the other end to the aluminum foil as shown. If your battery works, you should hear music from the electronic melody. If it doesnft work, try reversing the charcoal battery to find the correct positive and negative terminal. |
|
Note: If electronic melody is not available, you can use a multi tester instead. One advantage of this is you can measure the current flow and the voltage of your improvised battery. |





|
Option 1 |
|
· Salt (about 2-3 tbsp) · Water · Drinking Cup · Paper Towel · Charcoal · Aluminum Foil · Connecting wires with alligator clip · Electronic Melody |






|
MATERIALS |
|
PROCEDURE |